Generic Drugs: What They Are, Why They Work, and When They Don’t
When you pick up a prescription, you might see generic drugs, lower-cost versions of brand-name medications that contain the same active ingredient. Also known as generic medications, they’re required by law to work the same way as the original—same dose, same route, same effect. But here’s the catch: they don’t have to be identical in every way. The FDA says they’re bioequivalent, meaning they deliver the same amount of active drug into your bloodstream within the same timeframe. That’s true for most people. But for some, even tiny differences in fillers, coatings, or how the pill breaks down can make a real difference.
That’s why inactive ingredients, the non-medical parts of a pill like dyes, binders, or fillers. Also known as excipients, they’re not just harmless additives. For people with sensitivities, allergies, or conditions like celiac disease, these can trigger reactions—even if the active drug is perfectly matched. And when it comes to drugs with a narrow therapeutic index, medications where even small changes in blood levels can cause serious harm. Also known as NTI drugs, they include warfarin, levothyroxine, and some seizure meds, switching between generic brands can sometimes cause your levels to swing too high or too low. One study found that patients on levothyroxine who switched generics had noticeable changes in their TSH levels, even when the active ingredient was the same.
It’s not about quality—it’s about consistency. Brand-name drugs are made with the same formula, same factory, same batch process every time. Generics? They’re made by different companies, sometimes in different countries, using different processes. That’s why your doctor might ask you to stick with one brand of generic, or even stick with the original if you’re on a sensitive medication. It’s not that generics are bad. They’re often safe, effective, and save you hundreds a year. But they’re not magic clones. Your body might notice the difference, especially if you’re taking multiple meds, have a chronic condition, or are sensitive to changes in how things feel.
What you’ll find below are real stories and science-backed insights from people who’ve been there: why some switched from brand to generic and felt worse, how fiber can mess with absorption, what hidden animal ingredients are in pills, and why your thyroid med might need special handling. These aren’t theoretical debates—they’re lived experiences, backed by data, and focused on what actually matters: keeping you healthy, safe, and in control of your treatment.

How to Effectively Discuss Generic Medications with Patients
Learn how to clearly explain generic medications to patients, address common concerns, and improve adherence using proven communication strategies backed by clinical evidence and real-world data.
Read More
Coverage of Generics vs Brands: Key Policy Differences in Insurance Formularies
Insurance policies treat generic and brand-name drugs very differently, with generics costing up to 85% less. Learn how formularies, substitution rules, prior authorization, and state laws shape what you pay-and what you actually get.
Read More
Addressing Patient Concerns About Generic Medications: Common Questions Answered
Generic medications are just as safe and effective as brand-name drugs, but many patients still have questions. This guide answers the most common concerns about generics-look, cost, side effects, and when to stick with brand-name.
Read More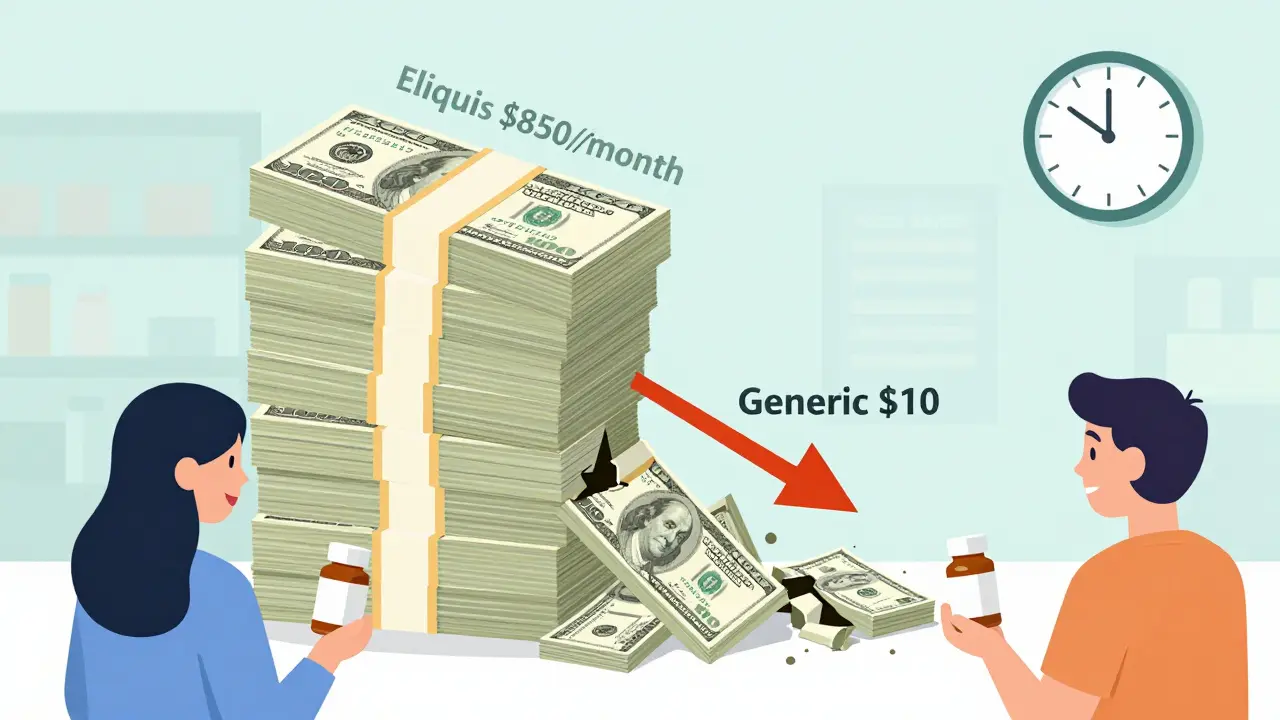
Economic Impact of Patent Expiration: When Drug Prices Drop
When pharmaceutical patents expire, drug prices drop dramatically-often by 80% or more. This article explains how generic competition drives down costs, why some drugs stay expensive despite patent expiry, and what patients can do to save money.
Read More
Provider Education on Generics: How Clinicians Can Improve Patient Outcomes and Cut Costs
Generic drugs make up 90% of prescriptions but many clinicians still doubt their effectiveness. Learn how provider education closes knowledge gaps, improves patient adherence, and saves billions in healthcare costs.
Read More
Bioequivalence Testing for Generic Drugs: What It Actually Proves
Bioequivalence testing proves generic drugs deliver the same active ingredient at the same rate and extent as brand-name versions. It's not guesswork-it's science backed by strict FDA standards and real-world data.
Read More
Medication Therapy Management: How Pharmacists Optimize Generic Drug Use
Medication Therapy Management helps pharmacists optimize drug use, reduce costs, and improve adherence-especially with generic drugs. Learn how this free service saves patients hundreds monthly and prevents dangerous medication errors.
Read More
Pharmacy Margin Economics: How Generics Drive Profits Despite Low Prices
Generics make up 90% of prescriptions but only 25% of drug spending-yet they generate 96% of pharmacy profits. This is how the math works, why independent pharmacies are closing, and who really benefits.
Read More
How to Verify the FDA Orange Book for Generic Equivalence
Learn how to use the FDA Orange Book to verify if a generic drug is truly equivalent to its brand-name version. Understand TE codes, AB ratings, and common mistakes to avoid unsafe substitutions.
Read More