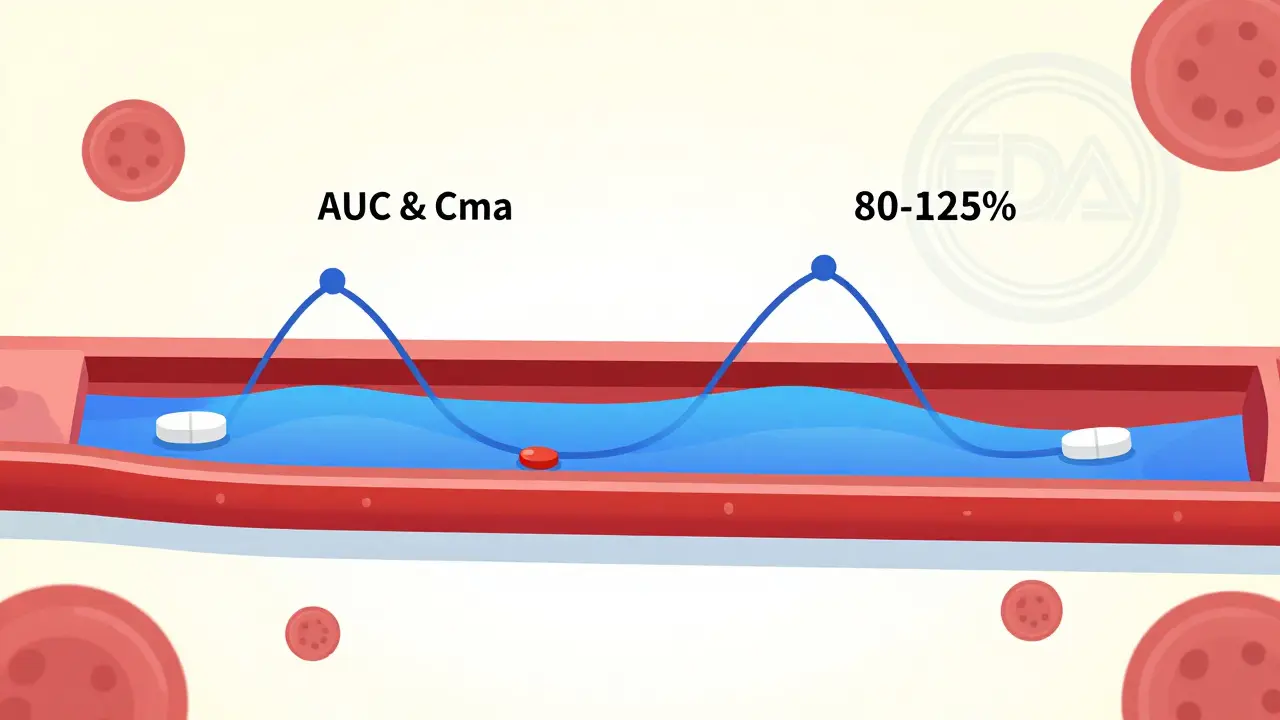
When you pick up a prescription at the pharmacy, you might see two options: the brand-name pill you’ve always known, or a cheaper generic version. You might wonder-does the generic really work the same? Is it just a copycat with lower quality? The answer lies in something called bioequivalence testing. It’s not marketing. It’s science. And it’s what makes generic drugs safe, effective, and legal to sell. Bioequivalence testing proves one thing, and one thing only: that a generic drug delivers the same amount of active ingredient into your bloodstream at the same speed as the brand-name version. It doesn’t test whether the drug cures your condition-that’s already been proven by the original brand. Instead, it answers a simpler, more critical question: Does your body get the same dose, the same way, every time? The U.S. Food and Drug Administration (FDA) requires this testing for every single generic drug approved in the country. The same rule applies in the European Union, Canada, Australia, and most major markets. Without passing bioequivalence, a generic drug can’t legally hit the shelves. So how does it work? The process starts with healthy volunteers-usually between 24 and 36 people. They take both the brand-name drug and the generic version, in random order, under strict conditions. Often, they fast beforehand to remove variables like food affecting absorption. Then, researchers take blood samples over several hours to track how much of the drug appears in the bloodstream and how quickly. Two key numbers matter: AUC and Cmax. AUC stands for area under the curve. It tells you the total amount of drug absorbed over time-basically, how much of the medicine your body gets. Cmax is the peak concentration-the highest level the drug reaches in your blood. That tells you how fast it gets absorbed. For a generic to be approved, both AUC and Cmax must fall within 80% to 125% of the brand-name drug’s values. That’s not a wide range. It’s tight. It means if the brand delivers 100 units of the drug into your blood, the generic must deliver between 80 and 125 units. That’s not a guess. It’s a statistical certainty, backed by data from dozens of people. This isn’t a loophole. It’s the result of decades of research. Before generics could be approved this way, patients had no guarantee. The 1984 Hatch-Waxman Act changed that. It created the Abbreviated New Drug Application (ANDA) process. Instead of repeating full clinical trials-which cost hundreds of millions-generic makers could prove equivalence through bioequivalence studies. The brand’s safety and effectiveness were already proven. The generic just had to show it performed the same in the body. And it works. Over 90% of all prescriptions filled in the U.S. are for generic drugs. That’s over 4 billion prescriptions a year. And yet, generics make up only about 23% of total drug spending. In 2020 alone, they saved the U.S. healthcare system $313 billion. That’s not a small win. That’s life-changing for people who can’t afford brand-name insulin, blood pressure meds, or antidepressants. Some people still worry. They say, “My generic made me feel different.” Maybe it did. But here’s the catch: it’s rarely because the active ingredient didn’t work the same. It’s usually because of the inactive ingredients-the fillers, dyes, or coatings. A generic might use a different dye, or a different type of starch to hold the pill together. For most people, that’s harmless. But for someone with a rare allergy or sensitivity, it might cause a mild stomach upset or a rash. That’s not bioequivalence failing. That’s a formulation difference. And pharmacists can often switch you to a different generic brand if that happens. What about complex drugs? Like inhalers, creams, or injectables? Those are trickier. You can’t just measure blood levels to know if an asthma inhaler is working right. The drug needs to reach your lungs, not just show up in your bloodstream. For those, regulators require different tests-sometimes clinical outcomes, sometimes specialized lab methods. The FDA has issued specific guidance for these cases, and the European Medicines Agency (EMA) requires extra studies for modified-release pills or topical products. Dr. Lawrence Yu, former deputy director at the FDA’s Office of Pharmaceutical Quality, put it plainly: “The FDA’s bioequivalence standards are among the most rigorous in the world.” And he’s right. The agency inspects over 1,200 generic drug factories every year-domestic and overseas-to make sure they follow strict quality rules. Every batch must meet identity, strength, purity, and stability standards. It’s not a free-for-all. You might hear myths: “Generics take longer to kick in.” “They’re weaker.” “They’re made in sketchy labs.” None of those are true for approved generics. A 2022 Consumer Reports survey of 1,200 users found 87% saw no difference between generic and brand versions. Nine percent even said the generic worked better. Only 4% reported worse results-and most of those were linked to side effects from inactive ingredients, not lack of potency. Still, 32% of patients in a 2021 study believed generics were less effective. That’s a perception problem, not a science problem. Misinformation spreads faster than facts. But the data doesn’t lie. If your doctor prescribes a generic, you’re getting the same medicine. The same active ingredient. The same dose. The same effectiveness. The future? More advanced tools. The FDA is now exploring computer modeling to predict how a drug behaves in the body. Instead of always testing in people, scientists might use physiologically based pharmacokinetic (PBPK) models to simulate absorption. That could speed things up for complex generics without lowering standards. The bottom line? Bioequivalence testing isn’t a shortcut. It’s a precise, science-backed method that ensures you get the same treatment whether you pay $10 or $100 for your pill. It’s why millions of people can afford their meds. It’s why generic drugs are trusted by doctors, pharmacists, and regulators worldwide. If you’ve ever doubted a generic, ask your pharmacist. They can show you the data. They’ve seen the studies. And they’ll tell you the same thing the FDA does: if it’s approved, it works.
How Bioequivalence Testing Works: Step by Step
Here’s what actually happens when a generic drug company wants to get its product approved:
- Pharmaceutical equivalence: The generic must contain the exact same active ingredient, strength, dosage form (tablet, capsule, etc.), and route of administration (oral, injection, etc.) as the brand-name drug.
- In vitro testing: Before testing in people, labs compare how quickly the generic dissolves in a lab solution versus the brand. This is called dissolution testing. If the profiles don’t match, the drug won’t absorb the same way in the body.
- In vivo study: A clinical trial with 24-36 healthy volunteers. Each person takes both the brand and generic, in random order, with a washout period in between. Blood samples are taken every 15-30 minutes for up to 24-48 hours.
- Statistical analysis: Researchers calculate AUC and Cmax for each person. Then they find the 90% confidence interval for the ratio between generic and brand. It must fall between 80% and 125% for both values.
- Regulatory review: The FDA reviews all data-dissolution, blood levels, study design, manufacturing details. If it passes, the generic gets approved.
What Bioequivalence Testing Does NOT Prove
It’s important to know what this test doesn’t cover:
- Long-term safety: The brand’s safety profile is already established. Bioequivalence doesn’t retest side effects that show up after years of use.
- Effectiveness for rare conditions: If the brand was tested on 5,000 patients with a rare disease, the generic doesn’t repeat that. It assumes the same effect based on bioequivalence.
- Local effects: For creams, inhalers, or eye drops, the drug’s action happens locally, not in the bloodstream. Blood levels don’t tell you if the cream penetrates the skin or if the inhaler delivers to the lungs properly.
- Manufacturing consistency over time: Bioequivalence is tested on one batch. But manufacturers must keep producing consistent batches under Good Manufacturing Practices (GMP), which are monitored through regular inspections.

Why Some Generics Still Raise Eyebrows
Even with solid science, skepticism lingers. Why? One reason is the “different pill” effect. If you’ve been taking a blue pill for years and suddenly get a white one, your brain thinks something’s changed-even if it’s the same medicine. That’s psychological, not pharmacological. Another issue is rare cases where patients switch between multiple generic brands. Each brand uses different inactive ingredients. If you switch back and forth, you might get inconsistent side effects. That’s not bioequivalence failing-it’s switching too often. Stick with one generic brand if you’re sensitive. Then there’s the myth that generics are made in “second-tier” factories. That’s false. The same companies often make both brand and generic versions. Many U.S. brand-name drugs are manufactured overseas, and so are generics. The FDA inspects them all the same way. And yes, there are exceptions. Drugs with a narrow therapeutic index-like warfarin, lithium, or levothyroxine-require extra caution. Even small differences in absorption can matter. For these, some doctors prefer to stick with one brand. But that’s a clinical decision, not a regulatory failure. The FDA still approves generics for these drugs, but they’re held to the same 80-125% standard, with additional monitoring.
Global Standards and the Future of Bioequivalence
It’s not just the U.S. The European Medicines Agency (EMA), Health Canada, and Australia’s TGA all use the same 80-125% range. The International Council for Harmonisation (ICH) helped align these rules globally. That means a generic approved in Australia meets the same standard as one approved in the U.S. The biggest shift coming? Modeling. Instead of always testing in people, scientists are using computer simulations to predict how a drug behaves. If the model shows the generic will behave like the brand, they might need fewer human studies. This is already being used for complex drugs like inhalers and long-acting injections. The FDA’s 2022 draft guidance on Model-Informed Drug Development for generics signals this change. It’s not about cutting corners-it’s about using smarter science. Less time in clinical trials. Faster access. Same safety.
What Patients Should Know
If you’re prescribed a generic:
- It’s not a cheaper version-it’s an equivalent.
- It’s been tested on real people, not just computers.
- It’s held to the same quality standards as the brand.
- It’s approved by the same agency that approved the original.
- It saves you money-often hundreds of dollars a year.