When you fill a prescription, the difference between a generic and a brand-name drug isn’t just in the price-it’s in how your insurance handles it. Many people assume generics are just cheaper versions of the same medicine. That’s true. But what most don’t realize is that insurance policies treat them like completely different products. The rules around who gets covered, how much you pay, and when you can even ask for the brand-name version are stacked heavily in favor of generics. And if you don’t understand those rules, you could end up paying hundreds more than you need to-or worse, stuck without the right medication.
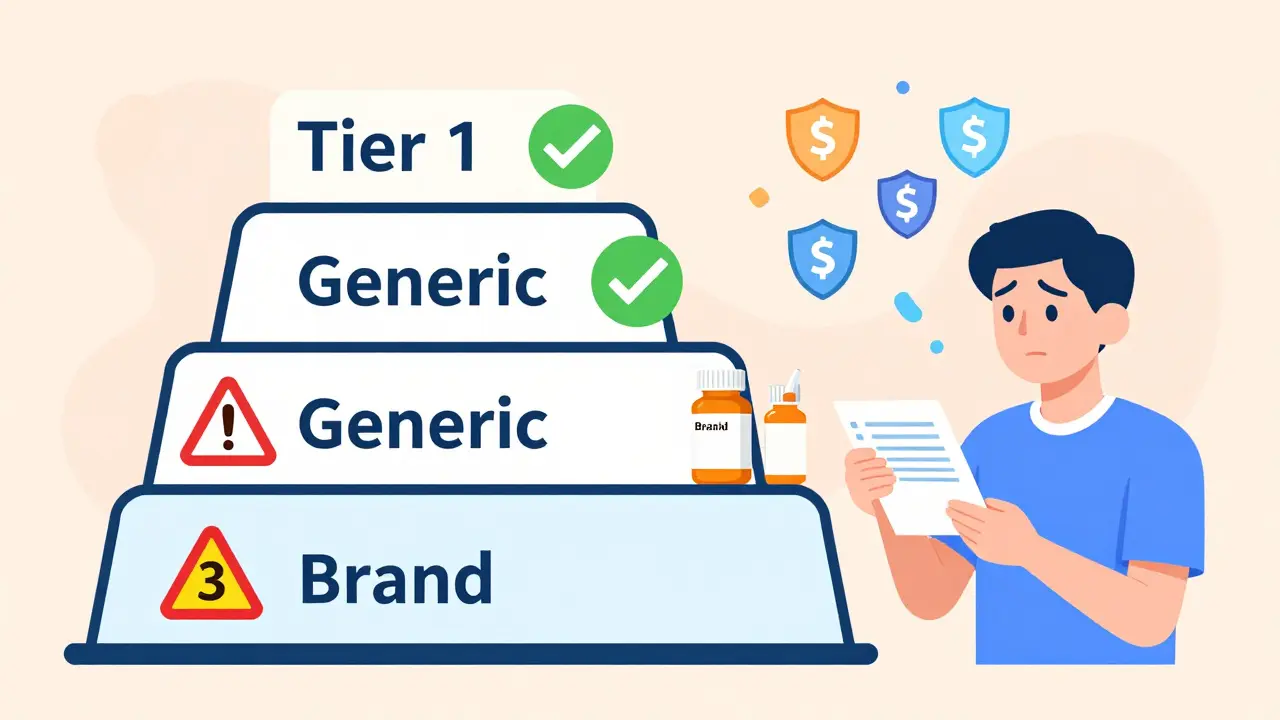
How Insurance Formularies Work: Tiers That Decide Your Costs
Insurance companies organize drugs into tiers. Think of it like a pricing ladder. The lower the tier, the less you pay. Generics almost always sit on Tier 1. That means a 30-day supply might cost you $5 to $15. Brand-name drugs? They land on Tier 2 or Tier 3. Copays jump to $40, $60, even $100. Some plans don’t even use fixed copays-they charge you a percentage of the drug’s full price, like 25% to 33%. That can mean paying $200 for a brand-name drug when the generic costs $10.This isn’t random. It’s by design. The whole system pushes you toward generics because they’re cheaper for insurers too. In 2022, 90% of all prescriptions filled in the U.S. were generics. But those generics only made up 23% of total drug spending. That’s how much money the system saves by favoring them.
Substitution Rules: The Pharmacist Has the Final Say
Here’s where it gets tricky. If your doctor writes a prescription for a brand-name drug-but a generic version exists-your pharmacist is legally allowed to swap it out. In every state, pharmacists can substitute generics unless the doctor specifically writes “Do Not Substitute” or “Dispense as Written.” That’s not a suggestion. It’s the rule.But here’s the catch: most doctors don’t write that. Why? Because they assume the generic is fine. And for most people, it is. But for some, it’s not. Patients with conditions like epilepsy, thyroid disease, or depression sometimes report different side effects or reduced effectiveness after switching. That’s not because the active ingredient changed-it didn’t. It’s because the fillers, dyes, or coating materials in generics can vary. And those can affect how the drug is absorbed.
What Happens When You Want the Brand?
If you ask for the brand-name drug when a generic is available, you don’t just pay the brand price. You pay the generic copay plus the full difference between the two. So if the generic costs $8 and the brand is $120, you pay $8 + $112 = $120. No discounts. No mercy. This is called “split payment,” and it’s used by Blue Cross Blue Shield, Aetna, and others to make the cost difference impossible to ignore.Medicare Part D does something similar. If you pick the brand over the generic, you pay 25% of the brand’s cost after hitting the catastrophic coverage threshold. That sounds fair until you realize the brand might cost $500 a month, and the generic is $12. Suddenly, you’re paying $125 instead of $3.

Prior Authorization and Step Therapy: The Bureaucratic Hurdles
Want to skip the generic and go straight to the brand? Good luck. Many insurers require prior authorization-meaning your doctor has to prove you need it. For brand-name drugs, 22.7% require this step. For generics? Only 2.1%. That’s a 10x difference.And then there’s step therapy. This rule says you have to try the generic first. If it doesn’t work, you move on. But “doesn’t work” isn’t defined clearly. For some conditions, like depression or chronic pain, insurers require you to try three different generics before approving the brand. That can take 6 to 8 weeks. During that time, your symptoms might worsen. And if you’re already struggling, that delay can be dangerous.
Medicaid, Medicare, and Commercial Plans: Who Gets What?
Coverage isn’t the same across all plans.- Commercial insurance: Generics cost $11.85 on average. Brands? $62.34. That’s over five times more.
- Medicare Part D: 91% of prescriptions are generics. But when you hit the coverage gap (the “donut hole”), you pay 25% of the cost whether it’s generic or brand. Still, brand-name drugs cost more upfront, so you hit the gap faster.
- Medicaid: Pays the lowest price possible for generics-87% lower than brand-name drugs. That’s because federal law forces manufacturers to give Medicaid their best price.
There’s also a twist: authorized generics. These are brand-name drugs made by the original company but sold as generics. They’re cheaper than the brand but often get better coverage than third-party generics because insurers trust them more.
When Generics Just Don’t Work
Not all drugs are created equal. Some have what’s called a narrow therapeutic index. That means even tiny differences in how the drug is absorbed can cause serious problems. Warfarin (a blood thinner), levothyroxine (for thyroid), and phenytoin (for seizures) fall into this category.Twenty-seven states have special rules for these drugs. They allow brand-name coverage without extra paperwork. But in other states, you still need to prove medical necessity. And proving that? It’s not easy. You need documentation from your doctor, lab results, maybe even a letter from your specialist. And even then, approval isn’t guaranteed.

What Patients Are Really Saying
On Reddit, one user posted: “I paid $85 for Crestor because my insurance denied my appeal for the brand. The generic gave me muscle pain I’d never had before.” That thread had over 1,200 upvotes. Another on Drugs.com said: “I switched from brand-name Lamictal to generic. My seizures doubled. Took 3 months to get my doctor to fight for the brand.”A Kaiser Family Foundation survey found 34% of insured patients didn’t understand when generics were covered. Nearly 1 in 5 avoided filling prescriptions because they feared the cost. And 44% of Medicare beneficiaries thought generics were less effective-despite FDA data showing they’re identical in active ingredients.
The Bigger Picture: Policy Shifts Coming
The FDA is updating labeling rules for generics starting in 2025. They’ll now clearly mark which generics are “therapeutically equivalent” to the brand. That’ll help insurers make better decisions.Medicare’s 2024 rule will force prior authorization decisions to be made within 72 hours for brand-name requests. Right now, approval times vary from same-day to two weeks. That delay can be life-threatening for some patients.
And then there’s the rise of specialty drugs-new, complex medications with no generic alternatives. In 2022, over half of new FDA approvals were in this category. That means insurance companies will have to rethink their entire substitution strategy. No generics? No cost savings. That’s going to change how policies are built.
What You Can Do
- Always check your plan’s formulary before filling a prescription. Many insurers have online tools.
- If you notice side effects after switching to a generic, tell your doctor immediately. Don’t wait.
- Ask your pharmacist: “Is there a generic available? If I take the brand, how much more will I pay?”
- If your doctor agrees the brand is necessary, make sure they write “Do Not Substitute” on the prescription.
- For Medicare users: Use the Medicare Plan Finder tool to compare coverage for your exact medications.
Generics save billions. That’s good. But they’re not perfect. And the system doesn’t always give patients the flexibility they need. Understanding your insurance’s rules isn’t optional-it’s essential to getting the right care without the wrong bill.
Are generic drugs really the same as brand-name drugs?
By law, generic drugs must contain the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. The FDA requires them to be bioequivalent, meaning they work the same way in the body. But inactive ingredients-like fillers, dyes, and coatings-can differ. For most people, this doesn’t matter. But for those with sensitivities or conditions like epilepsy or thyroid disease, those differences can affect how the drug is absorbed, leading to side effects or reduced effectiveness.
Why do insurance companies prefer generics so strongly?
Generics cost 80% to 85% less than brand-name drugs. In 2022, generics made up 90% of prescriptions but only 23% of total drug spending. That’s how much money insurers save. Lower costs mean lower premiums and copays for everyone. Insurers use tiered formularies, prior authorization, and step therapy to steer patients toward generics. It’s not about quality-it’s about economics.
Can I get my insurance to cover a brand-name drug if a generic exists?
Yes, but it’s hard. You need a medical necessity exception. Your doctor must document why the generic didn’t work-for example, side effects, therapeutic failure, or allergic reaction. Some states require three failed generic trials before approving the brand. Even then, approval isn’t guaranteed. Medicare and Medicaid don’t allow copay cards from drugmakers, so you’ll pay the full difference unless you qualify for financial assistance.
What’s the difference between a generic and an authorized generic?
A regular generic is made by a different company. An authorized generic is made by the original brand-name manufacturer but sold under a generic label. They’re chemically identical to the brand, often with the same inactive ingredients. Insurers often treat authorized generics more favorably because they’re trusted more than third-party generics. About 46% of all generic prescriptions in the U.S. are authorized generics.
Do Medicare and Medicaid cover brand-name drugs differently than private insurance?
Yes. Medicare Part D requires substitution unless medically necessary and has a coverage gap where patients pay 25% of the drug’s cost. Medicaid pays the lowest possible price for generics due to federal “best price” rules, making brand-name coverage harder to get. Private insurance often uses split payment (generic copay + price difference) to discourage brand use. Medicare and Medicaid don’t allow drugmaker copay cards, while private plans often do.
Why do some people have bad reactions to generic drugs?
The active ingredient is the same, but the fillers, binders, and coatings can vary. For drugs with a narrow therapeutic index-like levothyroxine, warfarin, or phenytoin-these differences can affect absorption. A 2022 JAMA Neurology study found 12.3% higher seizure rates in patients switched from brand to generic antiepileptics. Other patients report changes in side effects like nausea, dizziness, or fatigue. These aren’t random-they’re real, documented experiences that support the need for exceptions in coverage policies.
What should I do if my insurance denies coverage for a brand-name drug?
Ask your doctor to file an appeal with your insurer. They’ll need to submit documentation: lab results, records of failed generic trials, or evidence of side effects. Some insurers use a standard code like YN1 for medical necessity requests. If the appeal is denied, you can request an external review. You can also contact your state’s insurance commissioner or patient advocacy groups. In California, for example, law requires coverage if a generic causes an adverse reaction.
Are there any new policy changes coming in 2025?
Yes. Starting in 2025, the FDA will require clearer labeling on generic drugs showing their therapeutic equivalence rating. This will help insurers decide which generics are truly interchangeable. Also, CMS is enforcing a 72-hour rule for prior authorization decisions on brand-name drugs when generics exist. Right now, approval can take up to 14 days. These changes aim to reduce delays and confusion for patients.