Generic drugs are supposed to be the safe, affordable alternative to brand-name medications. They make up 92% of all prescriptions filled in the U.S. and save patients and the healthcare system billions every year. But behind the low price tag, a hidden risk is growing: contamination. In recent years, contaminated generics have led to cancer diagnoses, chemotherapy failures, and even fatal overdoses. This isn’t a rare glitch-it’s a pattern. And if you’re taking a generic drug, you need to know what’s going on and how to protect yourself.
What’s Actually Going Wrong in Generic Drug Manufacturing?
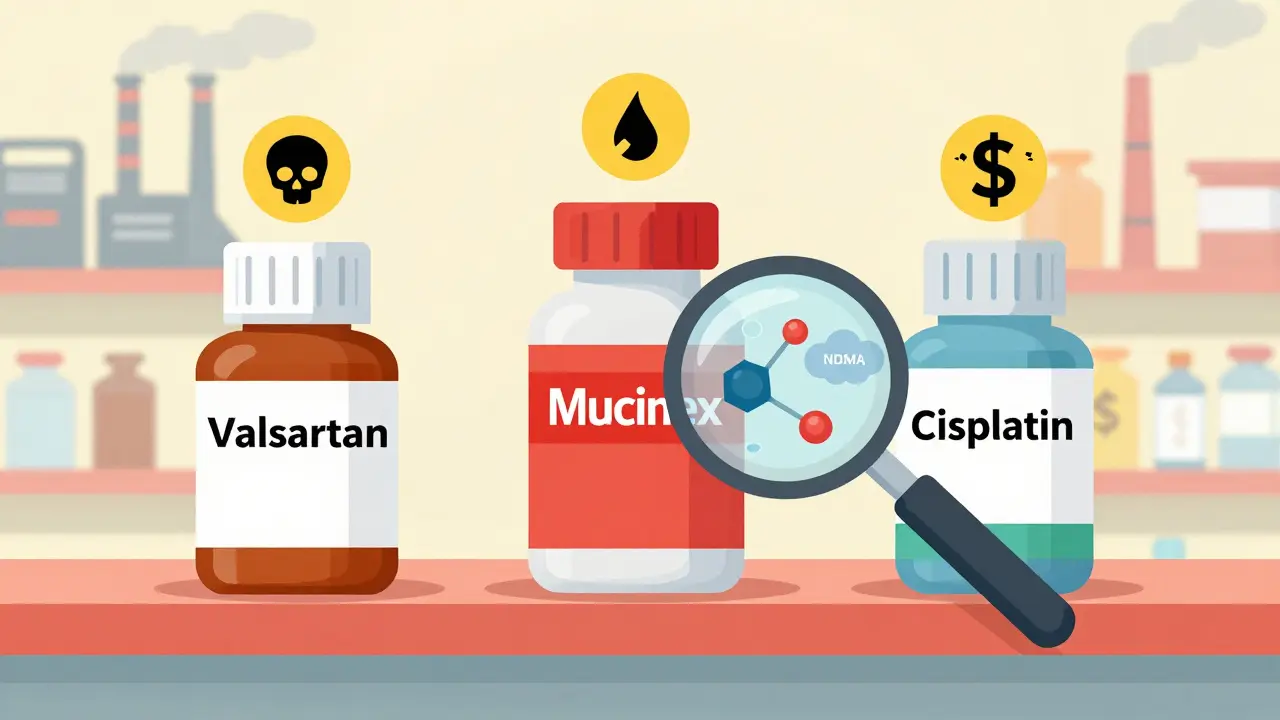
The problem isn’t that generics are inherently unsafe. It’s that the system designed to keep them cheap has created dangerous incentives. To get approval, manufacturers don’t need to run new clinical trials. They just need to prove their version works the same as the brand-name drug. That’s the Abbreviated New Drug Application (ANDA) process. But proving bioequivalence doesn’t mean checking for toxic impurities in the raw materials or the production environment. Take Valsartan, a common blood pressure medication. In 2018, the FDA found that some generic versions contained NDMA, a known human carcinogen. The contaminant showed up because a Chinese manufacturer, Zhejiang Huahai Pharmaceutical, changed their chemical process to cut costs. They added sodium nitrite, which reacted with other ingredients to form NDMA. Some batches had levels 200 times higher than the FDA’s safe limit of 96 nanograms per day. By 2025, over 1,300 lawsuits were filed by patients who developed cancer after taking the contaminated pills. One study found colorectal cancer rates were 7 times higher in exposed patients than in the general population. This wasn’t an isolated mistake. In 2025, a separate investigation found benzene-a known cause of leukemia-in over-the-counter Mucinex products sold by Walgreens. Independent lab tests showed some samples contained 4.7 parts per million (ppm), more than double the FDA’s 2 ppm safety limit. Patients who took these products regularly for over a year reported bone marrow damage, a classic sign of benzene poisoning.Chemotherapy Drugs That Don’t Work
The most terrifying cases involve cancer treatments. In 2025, STAT News reported that 17 chemotherapy drugs from Indian manufacturers failed basic quality tests. Twelve of them contained less than 80% of the labeled active ingredient. The FDA’s acceptable range is 85% to 115%. That means patients were getting a weaker dose-sometimes far weaker-than what their doctors prescribed. One facility, Intas Pharmaceuticals in Ahmedabad, was caught shredding quality control records and pouring acid on documents to hide failures. When inspectors visited in 2022, they found a “cascade of failure.” By spring 2023, 92% of major U.S. cancer centers reported shortages of critical chemotherapy drugs. Patients had to delay treatments or switch to more expensive brand-name versions. One case study at Memorial Sloan Kettering found that 7 out of 11 patients receiving contaminated cisplatin had no remission at all-despite having no other risk factors.Fentanyl Patches That Leak
Then there’s the opioid crisis. Between 2002 and 2025, over 52 million fentanyl patches were recalled because of seal failures. These patches are designed to release medication slowly over 72 hours. But when the seal breaks, the entire dose can leak out in minutes. In one 2023 recall by Sandoz, 0.8% of Duragesic patches leaked more than 15% of their intended dose. That’s enough to kill someone who’s never used opioids before. Emergency rooms reported multiple overdose cases tied to these faulty patches. One patient, a 68-year-old man with chronic pain, died after his patch leaked while he slept.
Where Are These Drugs Made-and Why?
Over 80% of the active ingredients in U.S. generic drugs come from China. India manufactures 40% of the finished pills and capsules sold here. These countries produce generics at a fraction of the cost of U.S. or European factories. But lower cost often means lower oversight. The FDA inspects foreign drug plants far less often than domestic ones. In 2025, only 13% of Indian manufacturing sites were inspected annually-even though they supply nearly half of America’s generic drugs. Meanwhile, the FDA has over 28,000 foreign facilities on its inspection backlog. In 2025, the agency had a budget of just $78 million for all foreign inspections. That’s less than $3,000 per facility per year. Some manufacturers have been flagged repeatedly. Zee Laboratories in India has received 46 FDA warning letters since 2018. In one 2024 inspection, inspectors found visible particles in every single vial of cisplatin they tested. The company didn’t fix the problem. They just kept shipping.What’s Being Done-and Why It’s Not Enough
The FDA has tried to fix things. In 2025, they launched the “Name Transparency Initiative,” promising to stop hiding drug names in inspection reports. For over a decade, the agency kept manufacturers’ identities secret, making it impossible for doctors and patients to know which brands were risky. Now, at least, you’ll be able to look up which company made your drug. They’ve also updated the Generic Drug User Fee Amendments (GDUFA III), requiring real-time testing for high-risk drugs like chemotherapy and blood pressure meds. But these rules only apply to new applications. They don’t fix the thousands of existing generic drugs already on shelves. Only 3% of Indian plants use continuous manufacturing-a process that reduces contamination risks by 78% according to MIT research. The problem? It costs $5 to $15 million to upgrade a facility. Most generic makers won’t pay it unless they’re forced to. The Drug Supply Chain Security Act (DSCSA) requires full electronic tracking of every pill by 2027. But right now, only 62% of U.S. pharmacies can verify where a drug came from. That means if a batch is contaminated, it could take weeks to trace it back-and in the meantime, people keep taking it.
How to Protect Yourself
You can’t control what’s in the bottle. But you can take steps to reduce your risk:- Check FDA recall lists weekly. Pharmacists surveyed in 2025 said they spend 22% more time verifying drug sources than they did in 2020. You can do the same. Visit fda.gov/drugs/drug-safety-and-availability/drug-recalls and search by drug name.
- Ask your pharmacist where your generic was made. If they don’t know, ask them to check. Some pharmacies now track manufacturing origin. If your drug came from a company with a history of violations, request a different batch or brand.
- Watch for changes in how the drug works. If you’re on levothyroxine and your TSH levels suddenly spike, or if your blood pressure medication doesn’t seem to be working, it could be a potency issue. Don’t assume it’s your body changing-ask for a new batch.
- Consider switching to brand-name for critical drugs. If you’re taking chemotherapy, blood thinners, or heart medications, the cost difference might be worth the safety margin. Some insurance plans cover brand-name drugs if you can prove the generic isn’t working.
- Report side effects. If you think a generic drug made you sick, report it to the FDA’s MedWatch program. Your report could help catch a pattern before more people are hurt.
What’s Next?
The Valsartan lawsuits are set to go to trial in late 2025. If courts find manufacturers knowingly hid contamination risks, it could change how the entire industry operates. Fines might rise. Penalties could become criminal. The FDA’s 2026-2030 plan promises AI-powered risk prediction and blockchain tracking-but those are still years away. For now, the system is still broken. Generics saved you money. But they also put you at risk. The question isn’t whether generics are safe. It’s whether you’re willing to gamble with your health for a few dollars.Are all generic drugs unsafe?
No, not all generic drugs are unsafe. Most are perfectly safe and effective. But the risk of contamination is higher in generics made by manufacturers with poor oversight, especially in countries where inspections are rare. The problem isn’t the generic label-it’s the manufacturing practices behind some of them.
How do I know if my generic drug was recalled?
Check the FDA’s drug recall page (fda.gov/drugs/drug-safety-and-availability/drug-recalls) using the drug name and manufacturer. You can also ask your pharmacist to verify the lot number on your bottle against current recalls. If you’re unsure, call the pharmacy and ask for the manufacturer’s name and country of origin.
Can I trust generics made in India or China?
Some are fine, others aren’t. Many Indian and Chinese manufacturers produce high-quality generics. But a significant number have been caught cutting corners. Companies like Zhejiang Huahai and Zee Laboratories have repeated violations. If your drug comes from a manufacturer with a history of FDA warnings, ask your doctor or pharmacist if a safer alternative exists.
Why doesn’t the FDA shut down these bad manufacturers?
The FDA can ban imports, but it can’t shut down factories overseas. They can issue warning letters, block shipments, or delay approvals-but they don’t have legal power to close facilities in other countries. Even when they find serious violations, penalties are often too small to deter repeat offenses. A $1 million fine means nothing to a company making $50 million in sales.
Should I stop taking my generic medication?
Never stop taking a prescribed medication without talking to your doctor. If you’re concerned, ask your pharmacist for the manufacturer’s name and check the FDA recall list. If your drug is on the list, your doctor can help you switch to a different generic or the brand version. Stopping suddenly could be more dangerous than the contamination risk.

Post A Comment