Movfor vs Other COVID-19 Antivirals
Movfor (Molnupiravir)
Works: Error-prone replication
Effectiveness: ~30% hospitalization reduction
Timing: Within 5 days of symptoms
Administration: Oral capsules
Eligibility: Adults ≥18 with risk factors
Paxlovid (Nirmatrelvir/Ritonavir)
Works: Protease inhibitor
Effectiveness: Highest viral load reduction
Timing: Within 5 days of symptoms
Administration: Oral tablets (with ritonavir)
Eligibility: Adults ≥12 with risk factors
Remdesivir
Works: RNA polymerase inhibitor
Effectiveness: Shorter recovery time
Timing: Within 7 days of symptoms
Administration: IV infusion
Eligibility: Hospitalized patients
Key Takeaways
- Movfor (molnupiravir) is an oral antiviral taken within five days of symptom onset; it works by causing error‑prone viral replication.
- Paxlovid (nirmatrelvir/ritonavir) offers the highest viral load reduction but requires a boosting agent and tight drug‑interaction checks.
- Remdesivir is an intravenous option used in hospitals; it shortens recovery time but needs infusion facilities.
- Effectiveness varies with vaccination status, age, and the circulating SARS‑CoV‑2 variant.
- Choosing the right drug depends on timing, severity risk, kidney/liver function, and potential drug interactions.
When it comes to oral COVID‑19 antivirals, Movfor is the brand name for molnupiravir, an antiviral pill approved for treating mild‑to‑moderate COVID‑19 in adults at risk of severe disease. With new variants still emerging in 2025, many patients wonder whether Movfor is the best option or if alternatives like Paxlovid or Remdesivir might work better. This guide breaks down how each drug works, who should take it, and where the trade‑offs lie, so you can make an informed decision without wading through medical jargon.
What is Movfor (Molnupiravir)?
Molnupiravir belongs to a class called nucleoside analogues. It mimics the building blocks of viral RNA, tricking the coronavirus into copying its genome incorrectly. The result is a cascade of mutations that render the virus non‑viable. In clinical trials, Movfor reduced the risk of hospitalization or death by about 30% when started within five days of symptoms.
Key attributes of Movfor:
- Form: Two 200mg capsules taken twice daily for five days.
- Administration: Oral, no need for injections or monitoring.
- Eligibility: Adults≥18years with mild‑to‑moderate COVID‑19 who are unvaccinated or have risk factors (age≥65, obesity, diabetes, heart disease, immunosuppression).
- Contra‑indications: Pregnancy, breastfeeding, and severe renal impairment (eGFR<30mL/min/1.73m²).
How does it work?
Once absorbed, molnupiravir is converted into its active ribonucleoside‑triphosphate form. The viral RNA‑dependent RNA polymerase (RdRp) cannot distinguish it from the natural nucleotides, so it incorporates the analogue during replication. Each incorporation adds a mistake, and after several rounds the viral genome accumulates lethal mutations - a process known as “error catastrophe.” Because the drug targets a step common to all SARS‑CoV‑2 variants, its activity remains relatively stable even as the virus evolves.
Major Alternatives on the Market
While Movfor was the first orally available antiviral, several other options have entered the scene:
- Paxlovid - a combination of nirmatrelvir (a protease inhibitor) and ritonavir (a pharmacokinetic booster). It cuts the risk of hospitalization by roughly 89% in high‑risk patients when started within three days.
- Remdesivir - an intravenous nucleoside analogue that inhibits the viral RdRp. Marketed as Veklury, it shortens recovery time in hospitalized patients but requires a 3‑day infusion.
- Lagevrio - another brand name for molnupiravir sold in the U.S. and Europe. Formulation and dosing are identical to Movfor.
- Sotrovimab - a monoclonal antibody given as a single IV infusion. Its effectiveness drops against newer Omicron sub‑variants, so it’s now a secondary choice.
- Antiviral medication - the broader category that includes all the drugs mentioned above, each targeting a different step of the viral life‑cycle.
Comparison Table
| Attribute | Movfor (Molnupiravir) | Paxlovid (Nirmatrelvir+Ritonavir) | Remdesivir (Veklury) | Lagevrio |
|---|---|---|---|---|
| Form | Oral capsules | Oral tablets | IV infusion | Oral capsules |
| Dosing schedule | 200mg×2times/day for 5days | 300mg×2times/day for 5days (with ritonavir) | 200mg IV daily for 3days | Same as Movfor |
| Mechanism | Error‑prone viral replication (nucleoside analogue) | Protease inhibition + pharmacokinetic boost | RdRp chain termination (nucleoside analogue) | Same as Movfor |
| Hospitalization reduction | ~30% (clinical trial) | ~89% (EPIC‑HR trial) | ~5‑7% (based on ACT‑T trial) | ~30% (mirrors Movfor) |
| Time to start | Within 5days of symptom onset | Within 3days (ideal 2days) | Within 10days for in‑hospital use | Within 5days |
| Key contraindications | Pregnancy, severe renal impairment | Severe drug‑drug interactions, liver disease | Severe renal or hepatic impairment | Pregnancy, severe renal impairment |
| Typical side effects | Diarrhea, nausea, dizziness | Altered taste, hypertension, diarrhea | Elevated liver enzymes, nausea | Same as Movfor |
Pros and Cons of Each Option
Movfor (Molnupiravir) - The most straightforward pill, no special diet or boosters needed. Ideal for patients who cannot take ritonavir or need an easy outpatient regimen. The downside is a modest reduction in severe outcomes compared with Paxlovid.
Paxlovid - Packs a powerful punch and is the current gold standard for high‑risk patients. However, ritonavir blocks many enzymes, so doctors must review all other medicines, which can be a hassle for poly‑pharmacy patients.
Remdesivir - Only option for patients already hospitalized who can receive IV therapy. It shortens time to recovery but does not dramatically lower death risk, and the infusion requirement limits use to clinical settings.
Lagevrio - Essentially the same as Movfor; the choice usually comes down to local availability and insurance coverage.
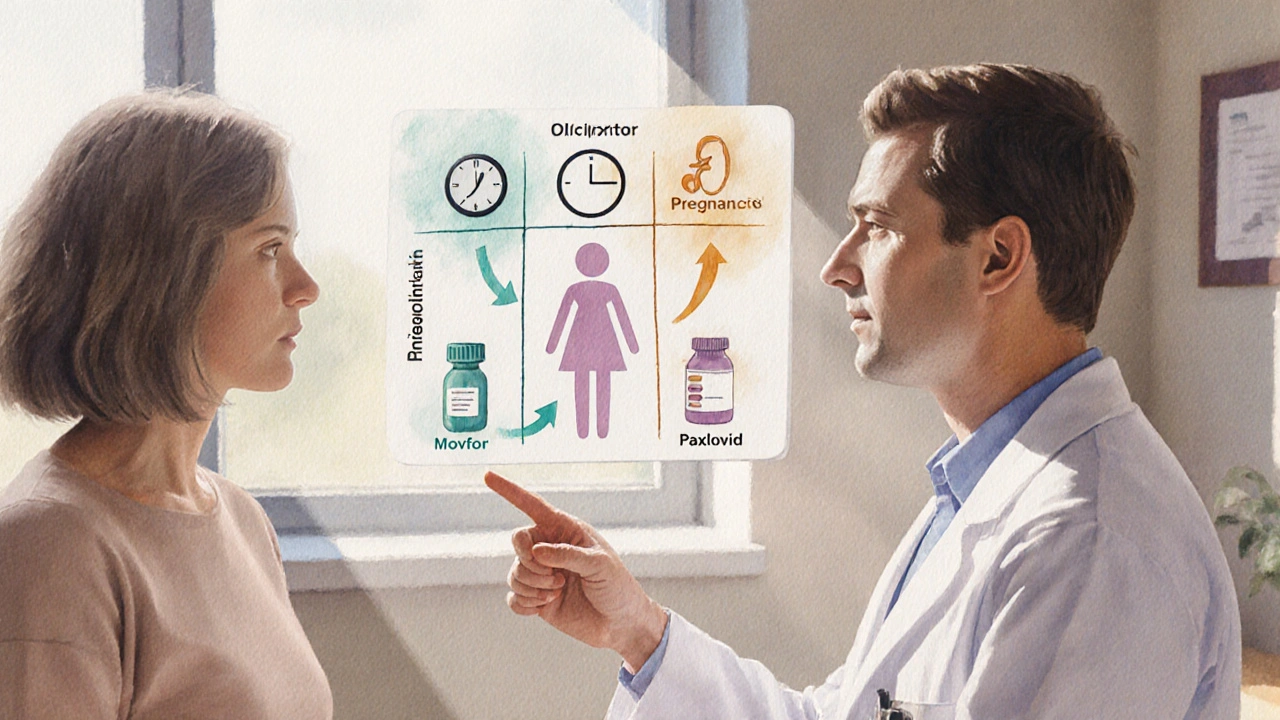
How to Decide Which Antiviral Fits You
- Timing is everything. If you can get treatment within 48hours, Paxlovid offers the best protection. If you miss that window but are still within five days, Movfor or Lagevrio become viable.
- Check your medication list. Ritonavir in Paxlovid interacts with statins, anti‑arrhythmics, and many antiepileptics. If you’re on any of those, discuss dose adjustments with a pharmacist or consider Movfor.
- Assess kidney and liver function. Severe renal impairment (eGFR<30) rules out both Paxlovid (due to ritonavir) and Remdesivir; Movfor may still be used if pregnancy isn’t a factor.
- Pregnancy status. Both Movfor and Lagevrio are contraindicated; Paxlovid is also not recommended. In such cases, monoclonal antibodies (if effective against the circulating variant) become the fallback.
- Hospital setting vs home. If you’re already admitted, Remdesivir is typically the default IV choice. For outpatient care, oral options dominate.
The most important thing to remember about Molnupiravir is that its benefit grows the earlier you start it, but it never reaches the efficacy levels of Paxlovid for the highest‑risk groups.
Potential Pitfalls and How to Avoid Them
- Missing the treatment window. Keep a rapid‑test kit at home and call your doctor as soon as symptoms appear.
- Skipping the booster dose. For Paxlovid, complete the full 5‑day course; stopping early reduces effectiveness.
- Ignoring drug interactions. Bring a current medication list to the pharmacy; many pharmacists run a computerized check for ritonavir.
- Assuming one size fits all. Variant‑specific data show that some antivirals lose potency against newer Omicron lineages; stay updated via your health authority.
Frequently Asked Questions
Can I take Movfor if I’m already on blood thinners?
Yes, molnupiravir does not have a known interaction with anticoagulants, so it’s generally safe. Always confirm with your prescriber.
How does Paxlovid’s ritonavir affect cholesterol meds?
Ritonavir can raise the level of statins like simvastatin, increasing the risk of muscle toxicity. Doctors usually switch to a low‑interaction statin (e.g., pravastatin) or pause the statin during treatment.
Is Remdesivir still used for mild COVID‑19?
No. Remdesivir is reserved for patients who need hospitalization because its IV delivery makes outpatient use impractical.
What should I do if I’m pregnant and test positive?
Both Movfor and Paxlovid are contraindicated in pregnancy. Discuss monoclonal antibody options or clinical‑trial enrollment with your obstetrician.
Do I need a follow‑up blood test after finishing an antiviral?
Routine labs aren’t required unless you experienced side effects affecting liver or kidneys. If you have underlying liver disease, a post‑treatment liver panel is prudent.
Next Steps
If you’ve just tested positive, follow this quick checklist:
- Note the date of symptom onset.
- Contact your primary care provider or tele‑health service within 24hours.
- Share a current medication list (including over‑the‑counter drugs).
- Ask specifically about Movfor, Paxlovid, or Remdesivir based on your risk factors.
- Arrange pharmacy pickup or infusion center appointment as directed.
Having this information ready speeds up the prescription process and ensures you get the most appropriate antiviral before the window closes.
Staying updated with the latest guidelines from the Australian Therapeutic Goods Administration (TGA) and the World Health Organization will help you navigate any new variants or drug approvals that appear later in 2025.


Post A Comment